Chemical elements constitute all of the matter around and us.
The term "element" is used for atoms with a given number of protons (regardless of whether or not they chemically bonded, e.g. hydrogen in water) as well as for a pure chemical substance consisting of a single element (e.g. hydrogen gas).
When different elements are chemically combined, with the atoms held together by chemical bonds, they form chemical compounds.
Only a minority of elements (about 32 elements) are found on Earth in native uncombined forms as relatively pure minerals, including copper, silver, gold, carbon (as coal, graphite or diamonds) and sulphur.
However, almost all the other elements are usually found on Earth in chemically combined forms, as chemical compounds and mixtures. For example, atmospheric air is primarily a mixture of nitrogen, oxygen, and argon, and native solid elements occur in alloys, such as the iron-nickel alloy.
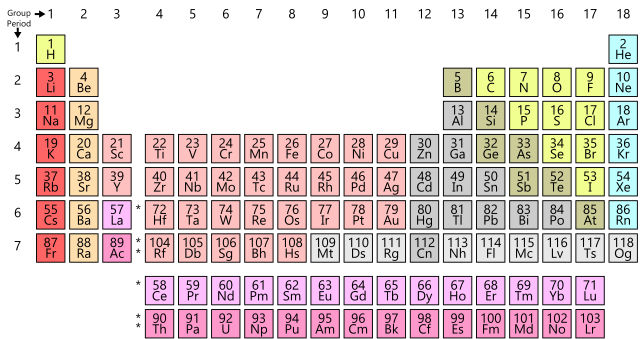
118 elements have been identified, of which the first 94 occur naturally on Earth with the remaining 24 being synthetic elements. They are listed in the Periodic Table.
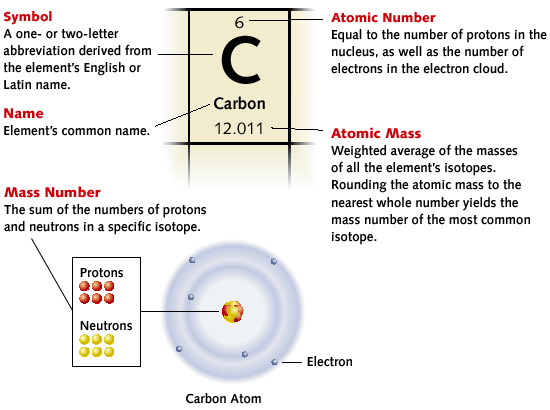
The Periodic Table is a way of listing the elements by the structure of their atoms. This includes how many protons they have as well as how many electrons they have in their outer shell. From left to right and top to bottom, the elements are listed in the order of their atomic number,which is the number of protons in their nucleus as well as the number of electrons in the electron cloud.
Each element has its own name and abbreviation in the periodic table. Some of the abbreviations are easy to remember, like H for hydrogen. Some are a bit difficult like Fe for iron or Au for gold. For gold the "Au" comes from the Latin word for gold: aurum".
It is called "periodic" because elements are lined up in horizontal rows from left to right according to their atomic number, and each row is called period. When they are lined up this way, elements in the columns have similar physical and chemical properties.
Each horizontal row in the table is a period. There are seven (or eight) total periods. The first one is short and only has two elements, hydrogen and helium. The sixth period has 32 elements.
Groups are the columns of the periodic table. There are 18 columns or groups and different groups have different properties.
One example of a group is the noble or inert gases. These elements all line up in the eighteenth or last column of the periodic table. They all have a full outer shell of electrons, making them very stable, so they tend not to react with other elements.
This lining-up and grouping of similar elements helps chemists when working with elements because they can understand and predict how an element might react or behave in a certain situation.
The periodic table was proposed by Russian chemist Dmitri Mendeleev in 1869. Using the table, Mendeleev was able to accurately predict the properties of many elements before they were actually discovered.
Here you have a song to learn the elements:
If you want to learn more about the elements in the periodic table that constitute all matter around us, have a look at this book:

No comments:
Post a Comment
Deja tus comentarios aquí:)